Observations of the blister beetle Stenoria analis (SCHAUM, 1859) |
© Hans Bahmer, 2017 |
1. Introduction |
|
In 1998, an artificial sandy xeric grassland with its typical flora was created on an area of about 60 square meters in the botanical garden of Gießen. For this purpose, the original soil was removed to a depth of about 80 centimeters and replaced with unwashed sand. In 2013, the author discovered the first (and to his knowledge still the only) known nesting colony of the ivy bee in Gießen in this habitat. In the meantime, more than 300 individual nests have been counted. The occurrence of the ivy bee in Gießen was already confirmed in 2008 by Dr. ULRICH FROMMER.  Fig. 1: Artificial sandy xeric grassland in the botanical garden of Gießen (photo 14.04.2016).
Fig. 1: Artificial sandy xeric grassland in the botanical garden of Gießen (photo 14.04.2016).
|
2. Development cycle of the ivy bee Colletes hederae |
|
The ivy bee which was only identified as a distinct species in 1993 by SCHMIDT and WESTRICH is one of approximately 560 wild bee species in Germany. Characteristic and eponymous for this species is the almost exclusive use of pollen of the ivy (Hedera helix) as larvae food. The larval development takes place underground in breeding chambers lined with silk made from a secretion (hence named "silk bees"). Due to the obligatory use of ivy pollen, this wild bee only appears from late summer to fall. In the observation area, bees were seen active from 16th August to 26th October.  Fig. 2: Development cycle of the ivy bee:
(a) a female ivy bee collects ivy pollen;
(b) a male ivy bee visits ivy to drink nectar and to mate with females;
(c) a female digs a nest; the excavation material is transported to the outside by the bee moving backwards;
(d) if a female shows up, numerous males appear to take their chance for mating; in the process, so called "mating balls" develop;
(e) a female transports pollen to its nest to fill the breeding chamber with pollen/nectar mixture; the larvae hatched from the deposited eggs will feed from pollen/nectar.
Fig. 2: Development cycle of the ivy bee:
(a) a female ivy bee collects ivy pollen;
(b) a male ivy bee visits ivy to drink nectar and to mate with females;
(c) a female digs a nest; the excavation material is transported to the outside by the bee moving backwards;
(d) if a female shows up, numerous males appear to take their chance for mating; in the process, so called "mating balls" develop;
(e) a female transports pollen to its nest to fill the breeding chamber with pollen/nectar mixture; the larvae hatched from the deposited eggs will feed from pollen/nectar.
|
3. Development cycle of the blister beetle Stenoria analis |
|
Stenoria analis is a cleptoparasite that has been shown to develop in the nests of ivy bees. After mating, females usually deposit their eggs on plants near ivy-bee colonies. The larvae hatching from the eggs are called triungulins and are characterized by their three-clawed feet (hence the name). It allows the beetle larvae to cling to male bees and to spread to the females during mating. With the females, the triungulins enter the bee nests where they develop at the expense of the bee offspring to the new beetle generation.  Fig. 3: Development cycle of the blister beetle Stenoria analis:
(a) mating activites (09.08.2017);
(b) oviposition on sea thrift (Armeria maritima) (13.08.2017);
(c,d) over time, changes of the eggs can be observed (28.08.2017, 01.09.2017);
(e) the beetle larvae have hatched and sit on the empty, now white-colored egg membranes (02.09.2017);
(f) the beetle larvae arrange themselves so that the brown-colored heads point to the outside (04.09.2017);
(g) if the cluster from (f) is deposited in an ivy bee colony, several males pounce on it and the larvae cling to the bees quickly (08.09.2017);
(h) a male ivy bee drinks nectar on northern hawkweed (Hieracium umbellatum); several larvae can be seen on its back (02.09.2017);
(i) mating of the ivy bees; the male is infested with triungulins (01.09.2016, photo: Annelies Polenz);
(k) female ivy bee with triungulin (03.09.2016).
Fig. 3: Development cycle of the blister beetle Stenoria analis:
(a) mating activites (09.08.2017);
(b) oviposition on sea thrift (Armeria maritima) (13.08.2017);
(c,d) over time, changes of the eggs can be observed (28.08.2017, 01.09.2017);
(e) the beetle larvae have hatched and sit on the empty, now white-colored egg membranes (02.09.2017);
(f) the beetle larvae arrange themselves so that the brown-colored heads point to the outside (04.09.2017);
(g) if the cluster from (f) is deposited in an ivy bee colony, several males pounce on it and the larvae cling to the bees quickly (08.09.2017);
(h) a male ivy bee drinks nectar on northern hawkweed (Hieracium umbellatum); several larvae can be seen on its back (02.09.2017);
(i) mating of the ivy bees; the male is infested with triungulins (01.09.2016, photo: Annelies Polenz);
(k) female ivy bee with triungulin (03.09.2016).
|
4. On the biology of the blister beetle Stenoria analis based on observations from the botanical garden in Gießen |
|
On 7th August 2016, the author observed for the first time a specimen of the blister beetle Stenoria analis in the colony of ivy bees mentioned above (Fig. 4a). Every day until 11th August 2016, a beetle appeared. When the author arrived, he was approached by the flying beetle before the bettle settled back into the typical vegetation (Fig. 4b). 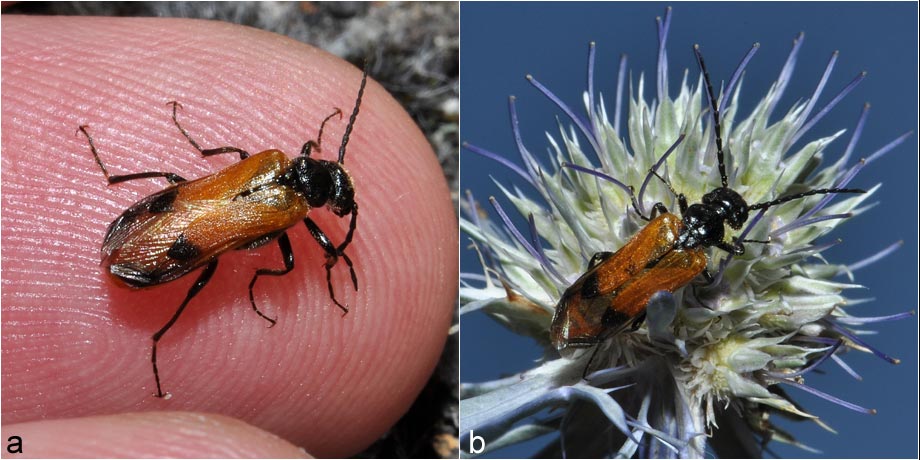 Fig. 4: (a) First discovery of Stenoria analis in the botanical garden of Gießen (07.08.2016); (b) beetle on the blossom of Eryngium planum (09.08.2016).
Fig. 4: (a) First discovery of Stenoria analis in the botanical garden of Gießen (07.08.2016); (b) beetle on the blossom of Eryngium planum (09.08.2016).
During the beetle activity period in 2017, more beetles were seen at the same time than could be counted (at least 14 specimens). This number of beetles settled on the author's cap, and there were also some around in flight. In the observation area, the beetles were active from 7th August to 29th August. MatingOn 9th August 2017, mating could be observed. The couple stayed in the lower vegetation (Fig. 3a). OvipositionFour ovipositionning events (Fig. 3b, 13.08.2017, Fig. 5a-c, 14.08.2017) were observed in 2017. Two clutches were found on the underside of the withered inflorescences of Armeria maritima (Fig. 3c). They were so well camouflaged that another one could only be found after the larvae had already hatched, although it was just a few inches away from the ones already known. One female laid its eggs on the still closed flower bud of Hieracium umbellatum (Fig. 5a, Fig. 5b) and another on pine needles (Pinus nigra, Fig. 5c). Thus, the clutches were at different heights: 30 cm, 80 cm and 200 cm.  Fig. 5: (a, b) Oviposition on northern hawkweed (Hieracium umbellatum) (14.08.2017); (c) oviposition on black pine (Pinus nigra) (14.08.2017);
Fig. 5: (a, b) Oviposition on northern hawkweed (Hieracium umbellatum) (14.08.2017); (c) oviposition on black pine (Pinus nigra) (14.08.2017);ClutchThe eggs are about a millimeter long, pale brown elongated structures that stick together and form the clutch. Depending on the surface, the clutch is more or less well camouflaged by its color (Fig. 3c, Fig. 5a-c). The author determined the egg count per clutch by counting the eggs visible from the outside on photos. This does not indicate the actual egg count, of course, but only a lower number, as not all eggs are visible in the photos. The clutches consisted of at least 117 eggs. In general, Stenoria analis is one of the species among the native blister beetles with the smallest clutches. During development, changes in the egg can be observed from the outside, increasingly easily with ongoing development, until the hatching of the beetle larvae (Fig. 3c-d). On 28th August 2017 the larvae from the clutch on pine needles hatched, on 2nd September, the ones from the clutch on sea thrift and on 6th September the ones from the clutch on the northern hawkweed. Thus, the development from egg to larva took between 15 and 24 days. 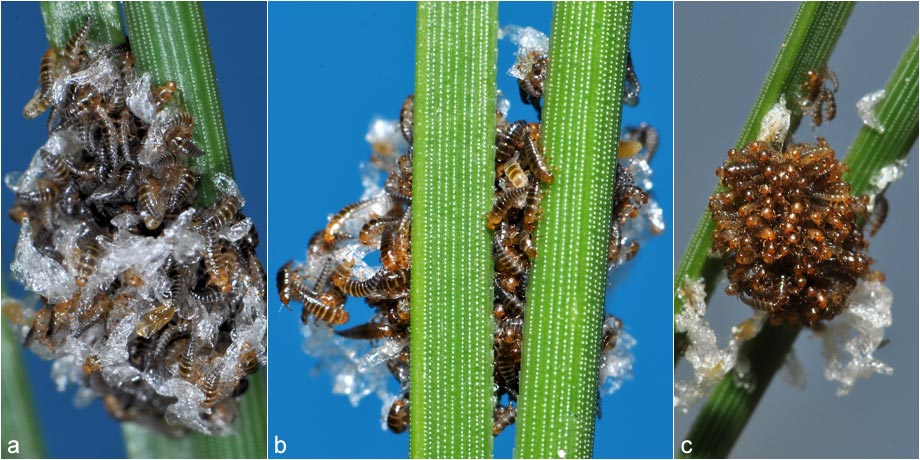 Fig. 6: (a, b) Larvae one day after hatching (29.08.2017); (c) arrangement of the larvae two days after hatching (30.08.2017).
Fig. 6: (a, b) Larvae one day after hatching (29.08.2017); (c) arrangement of the larvae two days after hatching (30.08.2017).LarvaeAfter hatching, the approximately one millimeter large beetle larvae (Fig. 7a), initially form a rather disordered lump (Fig. 3e, 6a-b) on which the empty egg shells are still visible. After one day, the larvae arrange themselves so that the brown heads point outwards (Fig. 3f, 6c). At this stage, the larvae are highly active and there is a strong but subtle movement of all the larvae. When touched with a brush, some of the animals immediately cling to the brush hairs. To cling to their host, the larvae use their feet. Each foot has a curved claw and two curved bristles, which is the origin of the name triungulin for this developmental stage (Fig. 7b). In addition, the larvae possess strong jaws (Fig. 7c), which are used when the larvae stick out vertically from their host and cannot use their claws (Fig. 3h).  Fig. 7: (a) Like all insects, the larval body is formed by the head with mouthparts and sensory organs, the breast with extremities attached and the abdomen; (b) eponymous claw; (c) head with strong jaws.
Fig. 7: (a) Like all insects, the larval body is formed by the head with mouthparts and sensory organs, the breast with extremities attached and the abdomen; (b) eponymous claw; (c) head with strong jaws.Finding the hostMovie 1: Triungulinen attach themselves to Colletes. © 2022 Beatrice Sippel, use with permission.
There is still no consensus about how the tringulins get into the host's nest. It is clear that the beetle larvae are transported by their hosts into the nests, because both male and female ivy bees with attached triungulins can be found (Fig. 3g-k). At the observation site in the botanical garden, the first contact of the larvae with their host certainly does not happen during the flower visit of nectar-seeking bees. None of the larval lumps was on a flower. The males of the ivy bees, hatching before the females, generally remain only a few centimeters above the ground in the colony during their search flights for females. If a female bee appears, a very violent reaction of the male bees usually occurs (Fig. 2d). In order to be "picked up" by the bees, the beetle larvae must attract attention. Since pheromones play a role in sex identification in honey bees as well as in wild bees, it is possible that blister beetle larvae have the ability to produce similar substances and thus attract their hosts (chemical mimicry). Another option would be optical mimicry. Here, the cluster of larvae with their brown heads facing outwards would try to fool the male bees into believing to see a female bee. The impression might be even reinforced by the movement of the beetle larvae, which could simulate a moving female bee to the males (Fig. 8a-c). In both baiting methods, only those beetle larvae are likely to have a good chance of being transported by male bees that appear before the female bees. Figures 8c, 8d and 8e show a larval group, which sits on the fruit stand of the hawkweed and was divided by its opening into smaller groups. Irrespective of whether the attraction of the host is due to chemical mimicry or optical mimicry, such a separation is likely to reduce the attractiveness. Thus, the question of why the ivy bees are tricked into approaching triungulin groups cannot be clarified by the author's observations and remains unclear (Figure 9a).  Fig. 8:
(a-c) Triungulin cluster on Armeria maritima; the group of larvae could mimic a colossal female bee (supernormal stimulus);
(d-f) triungulins sitting on the flower bud of hawkweed; by the opening of the bud they are split up into smaller groups.
Fig. 8:
(a-c) Triungulin cluster on Armeria maritima; the group of larvae could mimic a colossal female bee (supernormal stimulus);
(d-f) triungulins sitting on the flower bud of hawkweed; by the opening of the bud they are split up into smaller groups.
|
5. Special observation |
|
Stenoria analis was only found by the author in the Botanical Garden, because flying blister beetle regularly approached him. The beetles attempted to crawl into mouth and nostrils. When the author's chewing gum was stuck on his cap, 14 beetles gathered there. These probably were male beetles searching for females. The chewing gum might contain substances that signal the beetle males the presence of females (Fig. 9b).  Fig. 9:
(a) What attracts the males of the ivy bee? Visual cues or smell – that's the question!
(b) What attracts the males of Stenoria analis to the cap?
Fig. 9:
(a) What attracts the males of the ivy bee? Visual cues or smell – that's the question!
(b) What attracts the males of Stenoria analis to the cap?
|
6. Literature |
|


