First record of the tortoise beetle Cassida seladonia GYLLENHAAL, 1827 |
Text and photos © Tina Schulz, 2021 |
|
A warm day in early summer, a long foray through nature. Back at home, we find a caterpillar casually strolling over our clothes; a stowaway. Which nature lover hasn't experienced this?
Sometimes we are also amazed by the miracle of the appearance of an insect that was unintentionally introduced with collected substrate. Sometimes such guests are unwelcome, for example when they blatantly start feeding on the introduced substrate, or on the insects we want to rear with it. We usually hurry to detain the intruder, tear it off a strip and then compliment it out.  Fig. 1: Adolescent larva of Cassida seladonia, introduced into the garden with cudweed, observed on June 18, 2019. But sometimes such a chance find is just the beginning of something bigger. In the spring of 2019, I equipped a flowerbed in the garden with native plants that I gathered from the surrounding area. Among them was a slender cudweed (Filago minima): a very delicate, silvery plant with inconspicuous flowers that I had fetched from an oligotrophic grassland in the middle of Hanover (30 km away) – a very valuable biotope by local standards, but unfortunately abused as the city's largest dog toilet.  Fig. 2: First discovery of an adult Cassida seladonia on June 19, 2019 in Hanover, on strongly damaged slender cudweed (Filago minima).
On the evening of June 18, 2019, I discovered a beetle larva on it (see Fig. 1). Its typical "tail fork" (consisting of two long spinous appendages) laden with old skin remains and excrement, laying protectively over its body,
I recognized it as a shield beetle (Cassida sp.). The subsequent research brought the astonishing outcome, that it had to be Cassida seladonia, the cudweed tortoise beetle.
An narrowly oligophagous species, especially of cudweeds, less of Helichrysum and Gnaphalium, which has so far only been found in very few places in Germany
and is classified in category 1 on the Red List, i.e. is "threatened with extinction" [RHEINHEIMER & HASSLER, 2018].  Fig. 3: Feeding traces of Cassida seladonia on Filago minima. Visible larvae are marked with an arrow.  Fig. 4: Remarkable camouflage of young larvae of Cassida seladonia, which stay at the base of the host plant in resting phases.
The extreme lack of nutrients and lime, and the rapid drying out of the soil, which greatly slows down the scrub encroachment process, can also be attributed to the fact that there has never been any agriculture in the past.
Podsol is the predominant soil type. Due to the temporarily high level of groundwater, which is rich in iron and aluminum, the formation of bog iron ore takes place below the surface –
in contrast to the otherwise typical form of Podsol [LANDESHAUPTSTADT HANNOVER, 2001]. Specific plant species of the area include, for example,
silver birch (Betula pendula), creeping willow (Salix repens), common heather (Calluna vulgaris), matgrass (Nardus stricta), spike hairgrass (Aira praecox),
red sorrel (Rumex acetosella), heath dog-violet (Viola canina), maiden pink (Dianthus deltoides), sea pink (Armeria maritima ssp. elongata),
heath bedstraw (Galium saxatile), petty whin (Genista anglica), dwarf everlast (Helichrysum arenarium), Morison's spurry (Spergula morisonii), and the slender cudweed (Filago minima), the host plant of Cassida seladonia.  Fig. 5: Cassida seladonia larvae with increasing age. (a): Larva in the first stage (L1), recognizable by the fact that it hasn't yet had any stripped skin on its tail fork. (e): Due to the almost complete absence of feces, the stacking of the exuviae is exceptionally well visible in this specimen. It is therefore to be classified as L5 – freshly hatched, due to the still disproportionately large head. (f): Full-grown, now green larva in the last instar.
When two larvae were ready for pupation, they attached themselves head-down to the flower stem or to the inflorescence and got rid of their fecal shield (see Fig. 6). The first one pupated on June 24, 2019,
and the beetle emerged from the pupal shell on June 30, 2019 (see Fig. 7). This corresponds very well with the statement by RHEINHEIMER & HASSLER (2018),
according to which the new generation should hatch from July / August onwards.  Fig. 6: The last steps to the imago. (a): Full-grown larva; it no longer holds its fecal shield permanently over it in a protective manner. (b): Prepupa fixed upside down for pupation; it has already got rid of its fecal shield. (c): Pupa resting head-down on the plant stem. The last larval skin that was pushed together at the rear end during molting, differs in color from the similarly spiky lateral outgrowths of the pupa. (d): Pupal exuvia after the emergence of the beetle.  Fig. 7: Hatching of the adult beetle (the last few seconds). The elytra are still gaping at the tip, but only six minutes later they had assumed their final shape (see Fig. 8b1).  Fig. 8: The maturation process. Pictures (a1) and (b1) show a newly hatched (immature) Cassida seladonia. The fully colored, mature imago can be seen in (a2) and (b2).
When keeping the animals, an attempt was made to imitate the strong sun and heat exposure of their habitat as well as possible. I planted cudweed on sand in a transparent plastic cylinder that was twice as high as it was wide,
covered it with coarse gauze to allow for a good exchange of air and thus exposed it to the elements almost unprotected. 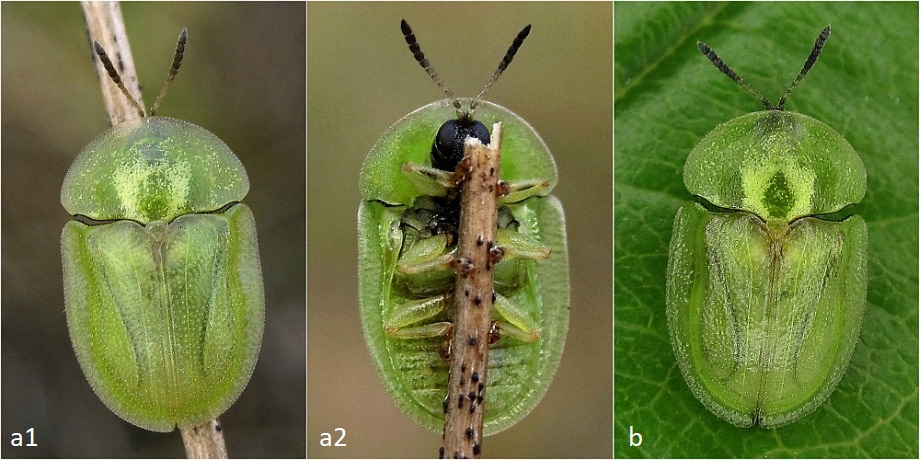 Fig. 9: In the prime of life. (a1) and (a2) show a flawless free-range find from June 21, 2019 (Hanover), (b) shows an equally beautiful breeding animal from June 30, 2019. After observing the inactivity of the beetles for a while, I decided to let them hibernate in the refrigerator – the local winter, which is more influenced by Atlantic climate, with its strong temperature fluctuations, especially on the southern side of the house, was too unsteady for me. To do this, I put the animals in a plastic Petri dish with a slightly moistened kitchen towel. They regularly changed their position in the vessel, but were immobile at every control. There was still no red coloration to be seen. Only their green color got a little warmer and reddish spots appeared on the shoulders; the edges of the elytra and pronotum also browned somewhat (see Fig. 10). 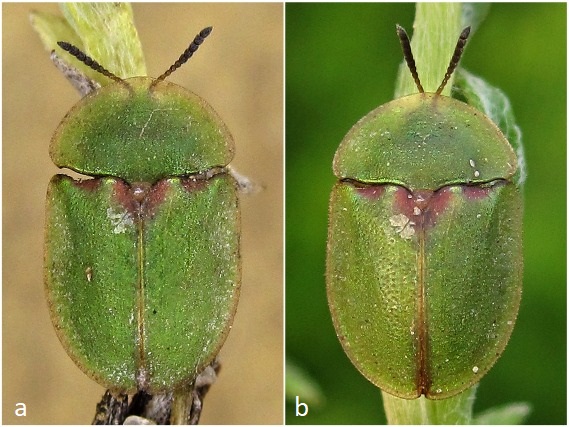 Fig. 10: This "old beetle", photographed on May 31, 2020 (a) or June 17, 2020 (b), had survived a whole year as a beetle: I saw it alive for the last time on July 7, 2020.
In mid-April 2020 I found out that two of the three beetles had not survived hibernation; I immediately put the third beetle outside, next to recently potted cudweed.
The survivor – who became active again after a week in the fresh air – still didn't think in the slightest about changing his green dress to bright red throughout. It stayed that way until its death; on July 7th, 2020
I photographed it alive last time. Impressively, the little one lived a full year, which was perhaps helped by the fact that it couldn't wear itself out in the strenuous reproductive business,
as it did not get the opportunity due to the lack of company after winter.  Fig. 11: No less than 27 larvae of Cassida seladonia were found at this point on the Kugelfangtrift (Hanover) on May 26, 2020.
Most of the larvae sat at the base of the plants, which were at most 5 cm high. At the time of the find (6–7 p.m.) some were busy eating. Most of them rested at the bottom of the cudweed stem and could easily be found
through the feeding marks described above – directly on the damaged plant or nearby. |
AcknowledgementsI would like to thank especially Dr. MICHAEL STERN from the University of Veterinary Medicine Hanover; he confirmed my initial identification on a specimen and gave me his time for a nice excursion into the biotope. Moreover, thanks are given to the rest of the kerbtier.de team for carefully confirming my identification of the first surprising larval find that was mentioned at the beginning. Last but not least, warm thanks are given to Dr. CHRISTOPH BENISCH, the webmaster of the extraordinary website kerbtier.de, for most of the translation of this article from German into English. |
Literature
|


